FDA Panel Rejects Avastin for Breast Cancer
Updated: 2011-06-30 05:35:09
Health News FDA Panel Rejects Avastin for Breast Cancer Print E-mail WEDNESDAY , June 29 HealthDay News The blockbuster cancer drug Avastin got soundly rejected late Wednesday as a treatment for metastatic breast cancer by a U.S . health advisory panel that found the medication was not effective and causes dangerous side effects . By unanimous vote , the panelists rejected the drug maker's appeal of a U.S . Food and Drug Administration recommendation last December to revoke Avastin's approval for breast cancer . The FDA recommendation cited the medication's poor performance in follow-up studies and its potential for serious side . effects The drug maker , Genentech , now owned by pharmaceutical giant Roche , was given an unusual two-day hearing this week before the six-member advisory .

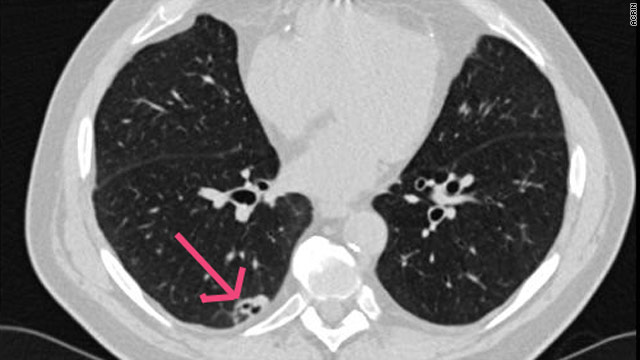 A heavy smoker for more than 45 years, Fernando Sandoval quit cold turkey after a CT scan revealed he had a tumor in his lung. Nine years ago Sandoval was one of the first people to participate in a clinical trial at UCLA to determine whether a low-dose CT scan can be used as a [...]
A heavy smoker for more than 45 years, Fernando Sandoval quit cold turkey after a CT scan revealed he had a tumor in his lung. Nine years ago Sandoval was one of the first people to participate in a clinical trial at UCLA to determine whether a low-dose CT scan can be used as a [...] An FDA advisory panel on Wednesday unanimously rejected arguments from Genentech that its controversial anti-cancer drug should continue as an approved therapy for advanced breast cancer.
An FDA advisory panel on Wednesday unanimously rejected arguments from Genentech that its controversial anti-cancer drug should continue as an approved therapy for advanced breast cancer. Subscribe to our blog Your : email About DNA Genotek Welcome to The Genetic Link a blog from DNA Genotek written to provide new insights about DNA and RNA sample collection . Join us for the latest news or learn more at www.dnagenotek.com Most Popular Posts Rinse , Swab or Spit What's the Real Source of DNA in Saliva 7 Simple Steps to Maximize DNA Yield with Orageneâ¢DNA Saliva-based DNA Collection Solves a Tropical Disease Mystery 3 reasons DNA from saliva is ideal for GWAS Lifestyle Management , Weight Loss and Genetics DNA saliva samples for SNP and CNV analysis on microarrays TLCâ s âLittle Coupleâ looks to DNA from saliva for genetic testing Introducing Performageneâ¢LIVESTOCK DNA Genotek's Newest Product DNA Genotek's Top 10 List for 2009 Epigenetic change and gene
Subscribe to our blog Your : email About DNA Genotek Welcome to The Genetic Link a blog from DNA Genotek written to provide new insights about DNA and RNA sample collection . Join us for the latest news or learn more at www.dnagenotek.com Most Popular Posts Rinse , Swab or Spit What's the Real Source of DNA in Saliva 7 Simple Steps to Maximize DNA Yield with Orageneâ¢DNA Saliva-based DNA Collection Solves a Tropical Disease Mystery 3 reasons DNA from saliva is ideal for GWAS Lifestyle Management , Weight Loss and Genetics DNA saliva samples for SNP and CNV analysis on microarrays TLCâ s âLittle Coupleâ looks to DNA from saliva for genetic testing Introducing Performageneâ¢LIVESTOCK DNA Genotek's Newest Product DNA Genotek's Top 10 List for 2009 Epigenetic change and gene It happens every year right around my birthday — the annual mammogram. It’s no big deal to me anymore, because I figure things are either going to turn out OK, or they’re not, in which case, I’ll know what to do, because I’ve done it before. So, I just submit to the routine, cross my [...]
It happens every year right around my birthday — the annual mammogram. It’s no big deal to me anymore, because I figure things are either going to turn out OK, or they’re not, in which case, I’ll know what to do, because I’ve done it before. So, I just submit to the routine, cross my [...] Subscribe to our blog Your : email About DNA Genotek Welcome to The Genetic Link a blog from DNA Genotek written to provide new insights about DNA and RNA sample collection . Join us for the latest news or learn more at www.dnagenotek.com Most Popular Posts Rinse , Swab or Spit What's the Real Source of DNA in Saliva 7 Simple Steps to Maximize DNA Yield with Orageneâ¢DNA Saliva-based DNA Collection Solves a Tropical Disease Mystery 3 reasons DNA from saliva is ideal for GWAS Lifestyle Management , Weight Loss and Genetics DNA saliva samples for SNP and CNV analysis on microarrays TLCâ s âLittle Coupleâ looks to DNA from saliva for genetic testing Introducing Performageneâ¢LIVESTOCK DNA Genotek's Newest Product DNA Genotek's Top 10 List for 2009 Epigenetic change and gene
Subscribe to our blog Your : email About DNA Genotek Welcome to The Genetic Link a blog from DNA Genotek written to provide new insights about DNA and RNA sample collection . Join us for the latest news or learn more at www.dnagenotek.com Most Popular Posts Rinse , Swab or Spit What's the Real Source of DNA in Saliva 7 Simple Steps to Maximize DNA Yield with Orageneâ¢DNA Saliva-based DNA Collection Solves a Tropical Disease Mystery 3 reasons DNA from saliva is ideal for GWAS Lifestyle Management , Weight Loss and Genetics DNA saliva samples for SNP and CNV analysis on microarrays TLCâ s âLittle Coupleâ looks to DNA from saliva for genetic testing Introducing Performageneâ¢LIVESTOCK DNA Genotek's Newest Product DNA Genotek's Top 10 List for 2009 Epigenetic change and gene I have a new book, and it’s called “Defeat Cancer: 15 Doctors of Integrative and Naturopathic Medicine Tell You How.” I grabbed this Connie Strasheim paperback from my mailbox one day just as I was taking my boys to flag football practice. In the car went the book, and when I finally plopped down in [...]
I have a new book, and it’s called “Defeat Cancer: 15 Doctors of Integrative and Naturopathic Medicine Tell You How.” I grabbed this Connie Strasheim paperback from my mailbox one day just as I was taking my boys to flag football practice. In the car went the book, and when I finally plopped down in [...] You know that Ricky Martin commercial, where the singer belts out a peppy happy birthday tune and states his wish for less cancer and more birthdays? Well, Ricky will be pleased to know that tomorrow — more than seven years after cancer — I get another birthday! And while the big 41 won’t take effect [...]
You know that Ricky Martin commercial, where the singer belts out a peppy happy birthday tune and states his wish for less cancer and more birthdays? Well, Ricky will be pleased to know that tomorrow — more than seven years after cancer — I get another birthday! And while the big 41 won’t take effect [...] Joey hung out with me at my cancer follow-up yesterday. The guy was by my side when he was 3 years old and I was diagnosed, and now, at 10, he’s still sticking around!
Joey hung out with me at my cancer follow-up yesterday. The guy was by my side when he was 3 years old and I was diagnosed, and now, at 10, he’s still sticking around! I’m a face of stupid cancer. You can be, too. Just visit facesofstupidcancer.tumblr.com and submit a photo + snippet of your story. Be sure to check out all the other young survivors while you’re there!
I’m a face of stupid cancer. You can be, too. Just visit facesofstupidcancer.tumblr.com and submit a photo + snippet of your story. Be sure to check out all the other young survivors while you’re there! It seems sorta silly of me to complain about my hair, because I have hair, and having hair is a whole lot better than not having hair. I should just suck it up and be OK with the fact that I got bangs back in January, but as much as I thought I would like [...]
It seems sorta silly of me to complain about my hair, because I have hair, and having hair is a whole lot better than not having hair. I should just suck it up and be OK with the fact that I got bangs back in January, but as much as I thought I would like [...]